By Ella Hopkins
This blog is one of three, explaining how the coronavirus makes us ill but at differing levels of complexity. This is very much inspired by the format of a brilliant YouTube series by WIRED, with their video explaining how CRISPR works being particularly well made.
This version is for those of you who’ve studied, or are studying, biological sciences at higher education level. (If that’s not you, you might want to try the Simple English or GCSE science level blog instead.)
Do you understand what viruses are and how they infect cells to cause disease, but find it difficult to get all the information you want to know about the novel coronavirus (SARS-CoV-2) in particular in one article? That’s what I found, so I have compiled some the current findings from literature into one short, easy to digest (hopefully!) section. Most articles have not yet been peer reviewed, so should you have any feedback on the content please feel free to write a comment and the article can be amended.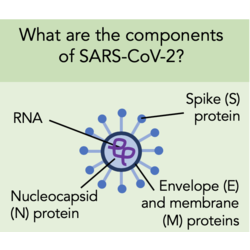
N.B. From now onwards I am going to call coronavirus by its proper name, SARS-CoV-2, to avoid any confusion with other coronaviruses. The coronavirus that caused the SARS outbreak is very similar to SARS-CoV-2, and is called SARS-CoV in this article.
SARS-CoV-2 is a (+)ss-RNA virus made up of four proteins; the spike (S) protein, envelope (E) protein, membrane (M) protein, and the nucleocapsid (N) protein. The S, E and M proteins make up the viral envelope, whilst the N protein holds the RNA inside the envelope. The spikes are the part of SARS-CoV-2 that binds cell membrane proteins in order to gain entry to cells. One of the cell membrane proteins that the spike protein has a high binding affinity for is ACE2.
Angiotensin converting enzyme 2 (ACE2) is a cell surface enzyme that cleaves angiotensin II into angiotensin 1-7. Angiotensin II is a vasoconstrictor, whilst angiotensin 1-7 is a vasodilator - so by increasing levels of angiotensin 1-7, ACE2 acts to lower blood pressure. There is another enzyme called ACE that catalyses the opposite reaction to increase blood pressure.
Once the S protein is bound to ACE2, the serine protease TMRPSS2 is required for cleavage of the spike protein to activate it. Activating the S protein is necessary for facilitating viral entry into the cell. Recent research has found that ACE2 and TMRPSS2 are required for viral entry into the cell, and well characterised TMRPSS2 inhibitors can block entry of SARS-CoV-2 into lung cell lines in vitro.
When SARS-CoV-2 binds ACE2 it is internalised into the cell. This means that fewer ACE2 proteins are present on the cell surface to help in blood pressure maintenance. (The virus may directly cause a reduction in the expression of ACE2, as seen with SARS-CoV, but this has not been proven yet with SARS-CoV-2 infection.) The balance then tips towards higher production of vasoconstrictors by the other enzyme ACE, which is still present at normal levels on the cell surface. Raising the blood pressure dangerously high can contribute to lung damage and potentially lead to severe acute respiratory failure. This could be the reason why having pre-existing high blood pressure is associated with worse clinical outcomes.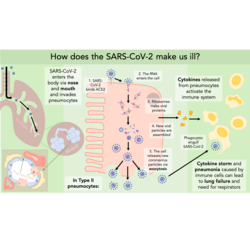
Pneumocytes are the main cell type infected by SARS-CoV-2 as they express both ACE2 and TMPRSS2. They are one of two cell types that make up the alveoli and are involved in the production of surfactant. Some infected cells die and so less surfactant is produced. The alveolar surface tension then increases and the alveolus collapses. Reduced efficiency of gas exchange due to collapsed alveoli can eventually lead to requiring hospital intervention and the use of respirators.
There is currently little information on the specifics of the immune response to SARS-CoV-2 infection as most publications are focussing on understanding viral entry to aid vaccine development and on clinical outcomes for patients. It can be expected though, that the response to SARS-CoV-2 is similar to that of SARS-CoV. Research on SARS-CoV has found that infected cells release interferons and pro-inflammatory cytokines and are responsible for initiating the innate immune response. It is still not known whether most of the lung damage observed in SARS-CoV-2 patients is due to a ‘cytokine storm’ response or directly by the virus infecting and killing lung cells. Hopefully, with the massive influx of literature on SARS-CoV-2 in the coming months, more will be found out and this article will be updated.
I hope you found this informative! Any feedback is very welcome – do write a comment and I will get back to you.
(To see that last graphic in more detail, use this link to a version on Twitter.)
