Forty years ago, a new rat poison called Vacor made headlines after being implicated in hundreds of human poisonings. A 48-year-old man’s legs became weak and numb two hours after ingesting Vacor. A 27-year-old developed diabetes shortly after ingesting Vacor, along with weakness, muscle aches, sleep disturbance and pain. Vacor was destroying pancreatic cells and producing distinct neurological impairments. In some cases, ingestion even led to death. Until recently, we did not know how Vacor was causing such catastrophic symptoms.
A decade later, researchers in Oxford were studying nerve regeneration in mice. To their great surprise, when they injured nerves in these mice their axons did not degenerate as expected—so the original research plan had to be abandoned but instead they had initiated an entirely new research field that we now know as programmed axon death.
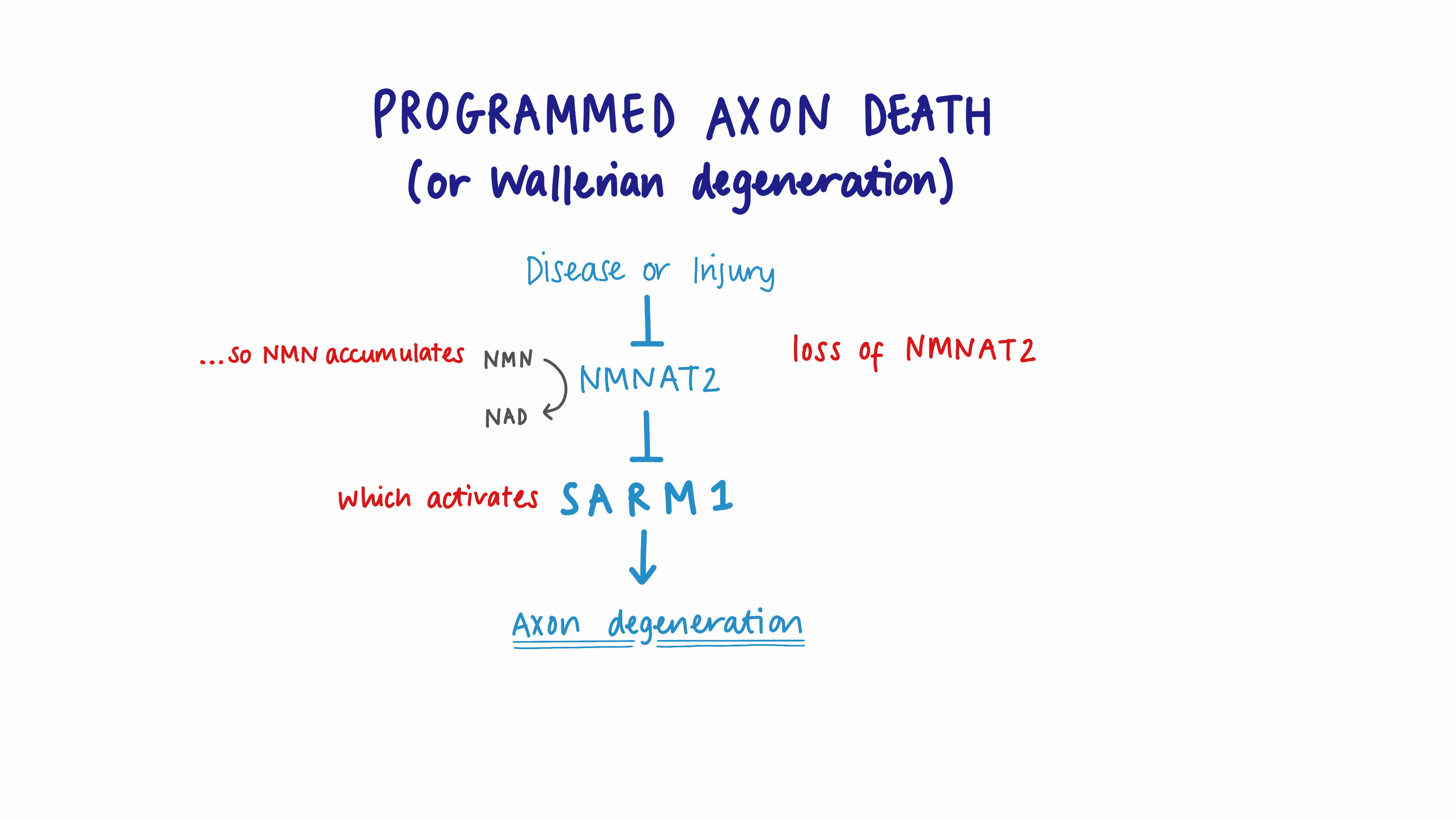
We subsequently identified the protective gene in these mice, and this knowledge allowed collaborators in Massachusetts to identify another protein with the opposite effect: it kills axons. This protein is known as SARM1. Today, blocking SARM1 is the basis of drug discovery programmes potentially valued at over $1 billion.
So what links Vacor to these unusual mice and drugs to treat axons?
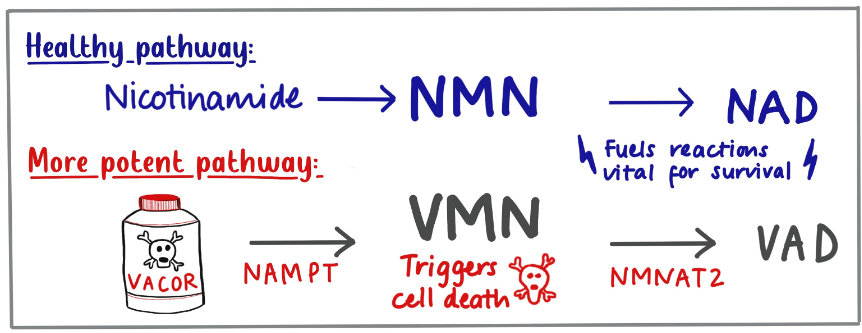
In 1980, the only known protection after Vacor ingestion was Vitamin B3 (or nicotinamide) if given quickly enough. The protein which protects mouse nerves (called NMNAT) works on molecules related to nicotinamide, so this suggests there could be some link, but what specifically?
Nicotinamide is converted inside cells to a molecule that activates SARM1 (called NMN). NMNAT keeps levels of this activator low. The Oxford mice had more NMNAT than normal so they keep it very low.
Our new report shows that when Vacor enters our cells, which it does very easily, it converts it to an even better activator of SARM1, one which no enzymes can stop. And indeed, all the early symptoms of Vacor poisoning do point to nerve degeneration. So solving a forty-year-old puzzle, the symptoms in humans, and its action as a rat poison, are almost certainly caused by SARM1.
So is Vacor all bad news? Well, not quite…
It turns out that mouse neurons lacking SARM1 are completely resistant to Vacor. We dosed them again…and again…and they ignored us and kept growing as if the Vacor wasn’t there. This is the second time we have seen indefinite axon protection when SARM1 is removed. Perhaps, somewhere out there, is a Vacor-resistant strain of rat lacking SARM1, inadvertently favoured over other rats by desperate but futile attempts to kill them with Vacor!
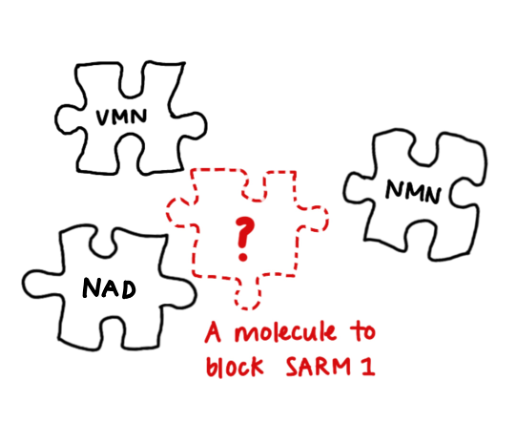
Even more exciting is how vacor can help develop new drugs to save axons. VMN is the third known allosteric regulator of SARM1, binding the same site as NMN and NAD, each with differing effects. Understanding how the structure produce these effects is now a strong basis for rational drug design.
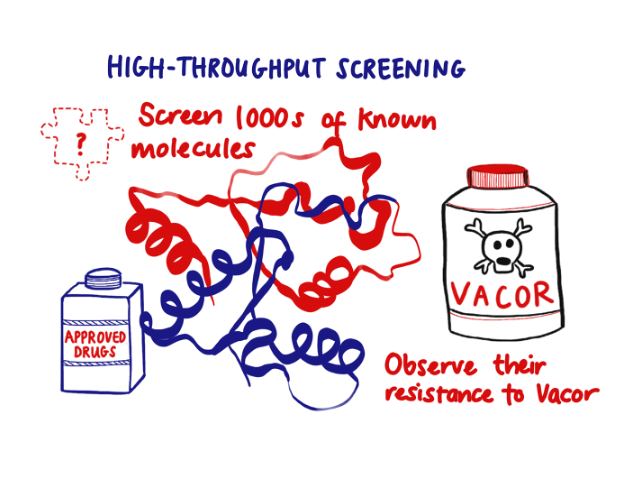
And the ease with which Vacor enters cells, which made it so dangerous, now becomes an advantage. High-throughput drug screens can simply add it to cultured cells to activate SARM1 very specifically. In this way, large drug libraries can be quickly screened to find inhibitors.
Research by many scientists over the last 40 years has finally shown why Vacor ingestion had such harmful consequences. While the damage inflicted to those individuals cannot be reversed, understanding the Vacor mechanism allows great strides to be made towards therapies which block SARM1 and now promise to benefit individuals with neurological conditions.